India is about one-third the size of the USA in terms of area. India is a pool of diverse population base of more than 1.2 billion and is a home for a numerous diseases, Institutions and hub of contract manufacturers and researchers. Indian economy stand as the third largest based on the Purchasing Power Parity (PPP) and in terms of globally eleventh largest by nominal Gross Domestic Product (GDP). India is today one of the top emerging markets in the global pharmaceutical scene. The sector is highly knowledge based and its steady growth is positively affecting the Indian economy. The organised nature of the Indian pharmaceutical industry is attracting several companies that are finding it viable to increase their operations in the country. Further, India is home to about 10,500 manufacturing units and over 3,000 pharma companies. India exports all forms of pharmaceuticals from APIs to formulations, both in modern medicine and traditional Indian medicines. This article provides a perspective on the Indian pharmaceuticals regulatory process from pre-independence era to till date.
Key words
CDSCO, Ministry of Health, DCGI, Subject Expert Committee, Regulatory process.
Introduction
Geographically, India is comprise area of 3.29 million sq. km. (1.27 million sq. mi.); about one-third the size of the USA. Genetically, culturally and socio-economicallydiverse population base of more than 1.2 billion is a home for a numerous diseases as well as for qualified, English-speaking professionals, Institutions and hub of contract manufacturers and researchers [
1,
2,
3].
Today, Indian economy stand as the third largest based on the Purchasing Power Parity (PPP) and in terms of globally eleventh largest by nominal Gross Domestic Product (GDP), due to its rapid growth, especially over the last decade, India is considered an industrialised nation. Apart from being a multi-ethnic, pluralistic society, India is also blessed with a variety of wildlife.
Indian Pharmaceutical Industry
McKinsey & Company a global management and consulting firm, through a major study it has reported that by 2020 India’s pharmaceutical sector will touch US$ 45 billion. The reasons for this optimism are well founded. In the period 2002–2012, the country’s healthcare sector grew three times in size, touching US$ 70 billion from US$ 23 billion. India's pharmaceutical market experienced a similar boom, reaching US$ 18 billion in 2012 from US$ 6 billion in 2005. The report further states that the Indian pharmaceutical market will be the sixth largest in the world by 2020.
The rise of pharmaceutical outsourcing and investments by multinational companies (MNCs), allied with the country's growing economy, committed health insurance segment and improved healthcare facilities, is expected to drive the market’s growth. India is today one of the top emerging markets in the global pharmaceutical scene. The sector is highly knowledge based and its steady growth is positively affecting the Indian economy. The organised nature of the Indian pharmaceutical industry is attracting several companies that are finding it viable to increase their operations in the country.
Further, India is home to about 10,500 manufacturing units and over 3,000 pharma companies. India exports all forms of pharmaceuticals from APIs to formulations, both in modern medicine and traditional Indian medicines [
4].
The Regulatory Process of India
The growth of pharmaceutical market in India has been very eye-popping during the last two decades.The growth of pharmaceutical market depends upon the various factors among one is drug regulatory system and regulatory legislations. Current scenario of drug regulatory system in India is on the way of continuous improvement and under transition phase viz., Strengthening of Central licensing agency, introduction of gazette notifications and amendment, formation of committees (SEC/NDAC, expert, technical and apex).
The history of drug regulatory dates back from British era, which time pharmaceuticals wereimported from abroad. Following the World War I, this situation has changed, and notonly were pharmaceutical products importedin increasing volume, but the demand forindigenouslydeveloped products also grew. However,many unprincipled foreign manufacturers flooded the Indian market with spurious and adulterated drugs andthis led to a rapid expansion ofpharmaceutical production during the earlypart of the century, and it became clear thatcomprehensive legislation was need of the hour. Hence, Government, then, formed a Drug inquiry committee under Sir Ram Nath Chopra also known as ‘Chopra Committee’ whose recommendations later on tabled amidst growing protest in legislative assembly as ‘The Drug Bill’ later on amended to the Drugs and Cosmetic Act 1940 (D and C Act) and Drugs and Cosmetic rules of 1945 [
5]. This would be the central legislation that regulates India's drug and cosmetic import, manufacture, distribution and sale. This also established the Central Drugs Standard Control Organization (CDSCO) [
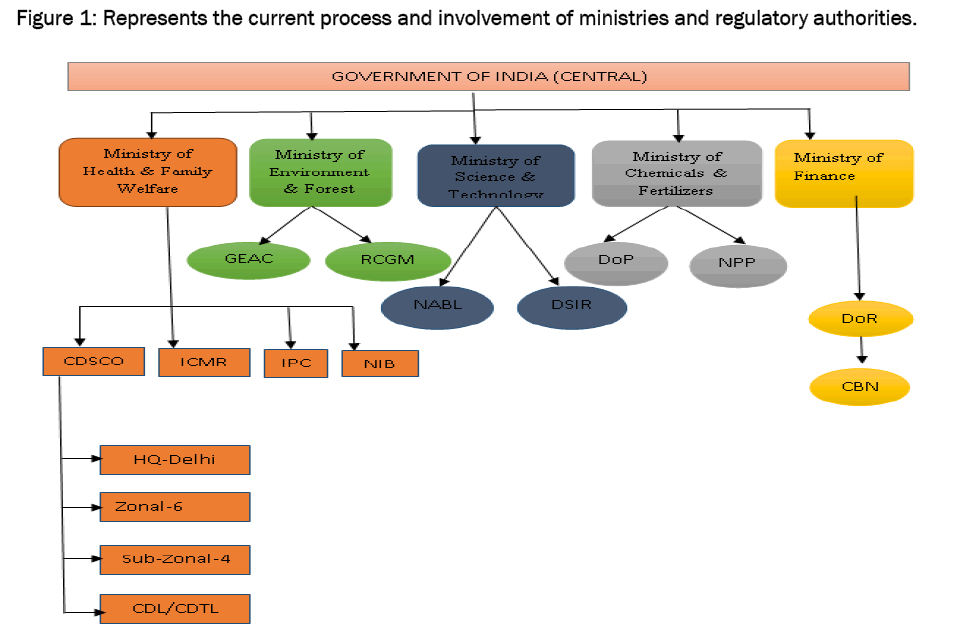
6]. The CDSCO works in the Directorate General of Health services, is a division in Ministry of Health and Family welfare, Government of India, headed by Drug Controller General of India (DCGI). At present CDSCO has six zonal offices, four sub-zonal offices, 11 port offices and sixlaboratories under its control. It has four zonal, three sub-zonal and seven port/airport offices and six laboratories to carry out its activities [
7].
Figure 1: Represents the current process and involvement of ministries and r.egulatory authorities.
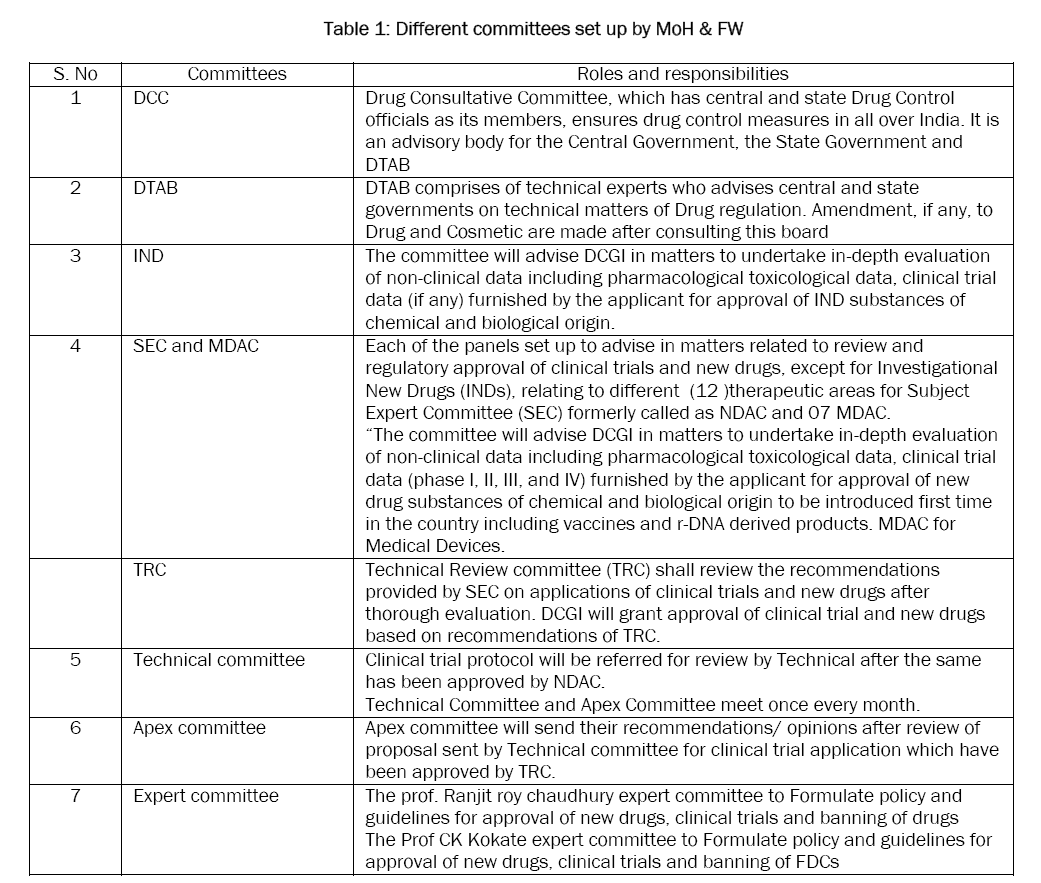
The various committees formed to facilitate the regulatory process and decision making of DCGI, a statutory board and a committee have been framed called Drugs Technical Advisory Board (DTAB) and Drug Consultative Committee (DCC) separately for Modern Scientific System of Medicine and Indian traditional system of Medicine and a provision of Central Drug Laboratory at Central Research Institute, Kasauli (HP) for testing of drugs. DTAB comprises of technical experts who advises central and state governments on technical matters of Drug regulation. Amendment, if any, to Drug and Cosmetic are made after consulting this board. Drug Consultative Committee, which has central and state Drug Control officials as its members, ensures drug control measures in all over India. It is an advisory body for the Central Government, the State Government and DTAB [
8-
16].
Table 1: Different committees set up by MoH & FW
The Indian government, realizing the potential of clinical research for new therapies, has modified and amended Schedule Y to the Drug and Cosmetics Rules of 1945. Schedule Y [
17] establishes a set of guidelines and requirements for clinical trials. However, Schedule Y was written with the generics industry in mind but increase entry of foreign pharmaceutical companies after the introduction of strict patent rules in the area of clinical research led the government to introduce many changes. The government recognized the importance of their regulation and thus developed Ethical and Regulatory Guidelines. The Indian Council of Medical Research (ICMR) issued the Ethical Guidelines for Biomedical Research on Human Subjects in 2000 [
18] and CDSCO released Indian Good Clinical Practice (GCP) guidelines in 2001 [
19].
Without a regulatory requirement for GCP compliance, however, most companies did not invest in clinical trials. Low quality data resulted in worsening India's reputation. Also, India's strict bureaucratic system made it hard to manage simple tasks like getting customs clearance for the equipment's. There were regulations which resulted in a phase lag, allowing companies to conduct a Phase II trial in India only if a Phase III study was going on somewhere else [
20]. However in 2005, CDSCO has come up with drastic revisions to Schedule Y to try to bring it on at par with internationally accepted definitions and procedures. The changes which took place were
1. Definitions for Phase I-IV trials, which eliminated the Phase lag [
21].
2. Clear responsibilities for investigators; and sponsors.
3. Requirements for notifying changes in protocol.
The Indian Government gave another boost to the drug-development industry by canceling the 12 percent service tax on clinical trials in 2007 [
22]. However, on Union budget presented 12 Jul 2014 chose to withdraw the service tax exemption provided on technical testing of new drugs, including vaccines and herbal remedies [
23]. In February 2009, the industry applauded new regulations on exporting samples. Previously, an export license was needed to get samples out of India but it has been removed now saving the time.
In order to further strengthen the scientific review and approval of new drugs/devices, the ministry has appointed 12 New Drug Advisory Committee’s (NDAC) and 7 Medical Device Advisory Committee’s (MDAC) to advise the CDSCO in making their decisions on approval of new drugs and global clinical trials, the NDAC expert committees have started reviewing the global clinical trial documents, from mid 2011 [
24].
As per the supreme court's directives, the Ministry of Health and Family Welfare (MoHFW) has off late come up with strong confidence building measures to protect the rights of the subjects participating in clinical trials (CTs), by notifying three back to back amendments to drugs and cosmetic rules namely Rule 122 DAB (first amendment) [
25], Rule 122 DAC (second amendment) [
26] and Rule 122 DD (third amendment) [
27]. Though these steps have been in the right direction, at the same time these have raised challenges for the pharma/device/biotech industry/Contract Research Organizations (CROs)/academic investigators and regulators themselves, all of whom have had to realign themselves with the requirements, which are now mandated. Investigators and their teams, the sites ethics committees (ECs) and the site/institutional heads/chairman all have got additional responsibilities as part of their scope. While, ECs have started applying and getting registered themselves. The unregistered ECs cannot legally review and accord their approval for CT protocols. This has led to delays in study initiation at those sites and crippled recruitment projections for approved CTs from the licensing authority (LA). Going forward, it would be advisable for sponsors/CROs to select sites, which are affiliated to registered ECs, rather than risking unregistered sites as part of the study. Approvals being issued for CT protocols have a binding on the applicant to abide with the new rules. This has triggered amendments to informed consent documents and their submission to ECs and LA. Changes in adverse event reporting requirements have forced investigators and ECs apart from the sponsors/CROs to be more vigilant and sensitive to the issue. They need to spend more time on each case than what they used to do in the past and report the event as per the defined process within the stipulated timelines. With time bound actions to be taken for serious adverse events (SAE) leading to CT related injury or death (including issues around compensation), all stakeholders need to have to proper systems in place to ensure compliance. There have been some debatable issues in Rule 122 DAB like provisions of compensation to be provided in case of failure of an investigational product to provide intended therapeutic effect (lack of efficacy); administration of placebo providing no therapeutic benefits or adverse effects due to concomitant medications, which need further clarification. Detailed risk assessment study plans need to be chalked out by sponsors to factor compensation related risks. Probability that people may get lured by economic incentives to participate as subjects in CTs have risen. The sponsors and insurance providers are revisiting the type and level of insurance and indemnity cover needed or which can be provided for the on-going and future CTs. The extent of documentation to be maintained at site, EC, sponsor, CRO and at LA end has grown multi-fold with these rulings.
Rule 122DAB which empowers the DCGI to determine the quantum of compensation that is to be paid to the family of the subject in case of a SAE in clinical trial. This amendment was introduced to allow a case by case review of the facts and circumstances that led to the SAE and accordingly determine the extent of negligence by each of the parties involved. Further to this, the CDSCO has now introduced a system to prescreening of all SAE reports that are to be considered by the DCGI. The prescreening will be done by CDSCO officers based on a prescribed checklist for determining the acceptability of an SAE report in order to ensure that it contains all necessary administrative and technical information necessary for ascertaining the nature and cause of the SAE, thereby allowing prudent determination of the quantum of compensation.
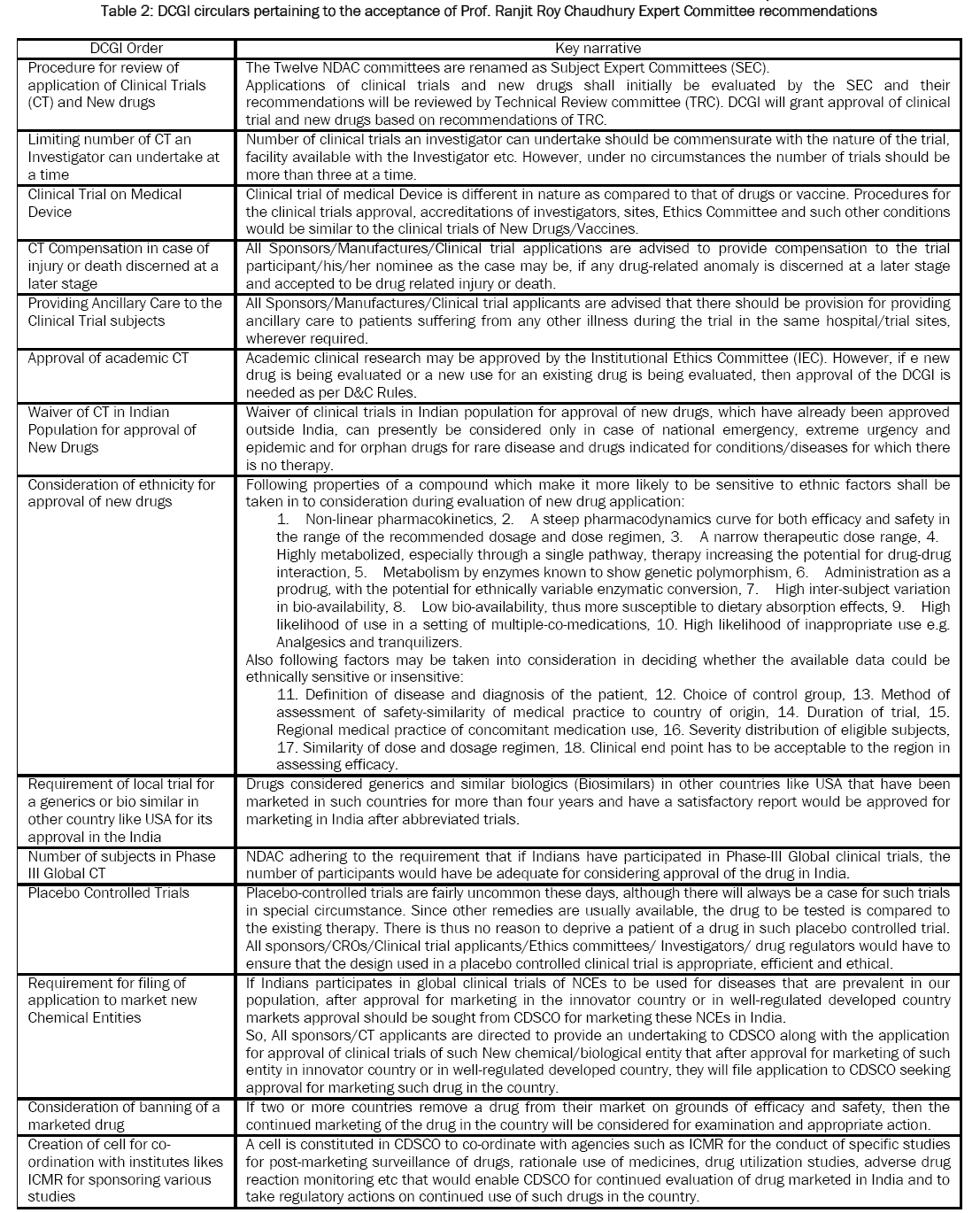
Table 2: DCGI circulars pertaining to the acceptance of Prof. Ranjit Roy Chaudhury Expert Committee recommendations
The expert committees constituted by the MoHFW in Feb 2013 for formulating guidelines, standard operating procedures and approval procedures have been provided recommendations upon receipt of opinion from stakeholders by holding formal meetings in order to come up with an effective policy document. The Recommendations by the six-member Prof. Ranjit Roy Chaudhury Expert Committee as, it suggested setting up of a council to oversee the accreditation of institutions, clinical investigators and institute ethics committees for clinical trials in the country. Stated clinical trials can only be carried out at accredited centers. Both the principal investigator of the trial, and the ethics committee of the institute should be accredited. Only those trials conducted at such centers should be accepted by the Drugs Controller General of India (DCGI).
Seeking to set up a Central Accreditation Council, the panel said the selection of assessors for accreditation and of experts to review new drug applications and other purposes should be made by a random procedure from a Roster of Experts.
Focusing on the importance of informed consent from each participant for clinical trials, the panel said any departure or violation from the approved process should result in blacklisting of the Principal Investigator for at least up to 5 years.
In circumstances where special groups of people who have diminished capacity to protect their interests are involved, the guardian can give consent and this should be witnessed by an independent person who also has to sign the document. Audiovisual recording of the informed consent process should be undertaken and the documentation preserved, adhering to the principles of confidentiality, it said.
Further, in its report states for replacement of the existing 12 drug advisory committees by a single broad expertise-based Technical Review Committee to ensure speedy clearance of applications without compromising on quality of data and rules and regulations.
On compensation for adverse effects (AE) or serious adverse effects (SAE) during trial, the committee puts the onus of responsibility on the sponsor investigator for providing medical treatment and care to the patient at his/their cost till the resolution of the AE/SAE. This is to be given irrespective of whether the patient is in the control group, placebo group, standard drug treatment group or the test drug administered group.
However, it does not favour paying of compensation for injury or death due to totally proven unrelated causes. In all other cases of death or injury/disability, compensation should be paid to the participant or his legal heirs [
28].
The MOH & FW has discussed and the recommendations of the expert committee were discussed in a meeting with its members. During the meeting, clarifications on certain recommendations were obtained from the committee. After the meeting, the ministry in-principle accepted the recommendations of the committee [
29].
The Drugs and Cosmetics (Amendment) Bill, 2013 [
30,
31]:
• The Drugs and Cosmetics (Amendment) Bill, 2013 was introduced in the Rajya Sabha on August 29, 2013. The Bill amends the Drugs and Cosmetics Act, 1940 and changes the name of the Act to the Drugs, Cosmetics and Medical Devices Act, 1940.
• The Bill proposes changes in the regulation of the import, export, manufacture, distribution and sale of drugs, cosmetics and medical devices and to ensure safety, efficacy, quality and conduct of clinical trials.
• The definition of drugs is changed to include new drugs that are (i) not in significant use in India and are not recognised as effective and safe by the Drugs Controller General of India (DCGI);(ii) approved by the DCGI for certain claims but are being marketed with modified/new claims; (iii) a fixed dose combination of two or more drugs, which are individually approved but are being combined for the first time in a fixed/changed ratio; and (iv) all vaccines, Recombinant Deoxyribonucleic Acid derived products, Living Modified Organisms, stem cells, gene therapeutic products etc. which are intended to be used as drugs.
• Under the Act, medical devices were covered under the definition of drugs. The Bill changes this by adding a definition of medical devices to include any instrument, implant, material or other article, including the software, intended to be used specially for human beings or animals for the specific purposes of diagnosis, prevention, treatment or alleviation of any disease or, injury, modification of the body’s anatomy and sustaining life.
• Clinical trials are defined in relation to drugs, cosmetics and medical, and involve their systematic study with the objective of determining their safety, efficacy, performance or tolerance. Anyone initiating a clinical trial has to register with the Central Drug Authority (CDA) and get approval from an Ethics Committee registered with it. The Bill creates provisions for the medical treatment and compensation in case of injury or death of a person during participation in a clinical trial or due to it.
• The Central Government shall establish a CDA to subsume the existing Central Drugs Standards Control Organisation. The CDA will be composed of representatives from the Ministries of Health and Family Welfare, Law, Commerce and Industry, Science and Technology, Chemicals and Fertilisers, DCGI, Indian Council of Medical Research, Directorate General of Health Services, and other experts nominated by the central government, including those from state licensing authorities.
• The CDA shall among others, specify guidelines, structures and requirements for the effective functioning of the central and state licensing authorities; review, suspend or cancel any licence or permission issued by them; and decide on disputes between two or more state licensing authorities relating to the provisions of the Act and rules and regulations made under it.
• The DCGI is the central licensing authority that has the power to issue, renew, suspend or cancel licences for import, export or manufacture of drugs, cosmetics or medical devices or permission for conducting clinical trials. The DCGI also has the sole power to issue licenses for the manufacture, sale, and export of 17 categories of drugs.
• The Bill constitutes the Medical Devices Technical Advisory Board and the Drugs Technical Advisory Board to advise the central and state governments and the CDA on technical matters pertaining to medical devices, and drugs.
In order to ensure standard quality of drugs, cosmetics, and medical devices, the Bill specifies conditions under which they will be considered misbranded, adulterated, and spurious and specifies penalties and offences for the same.
The pending Drugs and Cosmetics (Amendment) Bill, that seeks to centralise the licensing in key categories and establish the central drug authority (CDA), was not tabled for passage in the Parliament during the brief winter session of the House, till date [
32].
On 03 Jul 2014, DCGI has issued circulars pertaining to the acceptance of Prof. Ranjit Roy Chaudhury Expert Committee’s following recommendations mentioned in
Table 2 [
33].
References
- http://www.rlc.dcccd.edu/ibt/IntroductiontoIndia.pdf
- http://www.indiaonlinepages.com/population/india-current-population.html
- http://www.ibef.org/economy/indiasnapshot/facts-about-indian-economy
- http://www.ibef.org/industry/pharmaceutical-india.aspx
- Singh H. Sir Ram Nath Chopra: A profile. J Young Pharm. 2009;1:192–4.
- http://www.cdsco.nic.in/Drugs&CosmeticAct.pdf .
- http://www.cdsco.nic.in/forms/contentpage1.aspx?lid=1853
- http://www.medindia.net/indian_health_act/drugs.and.cosmetics.act.1940.the.drugs.consultative.committee.htm .
- Narrain S. A costly prescription.Frontline. 2005;22:12–25.
- Damodaran AD. Indian Patent Law in the post – TRIPS Decade: S and T Policy Appraisal. J Intellect Prop Rights. 2008;13:414–23.
- Tulasi GK, Rao BS.A detailed study of patent system for protection of inventions. Indian J Pharm Sci. 2008;70:547–54.
- Saha CN, Bhattacharya S. Intellectual property rights: An overview and implications in pharmaceutical industry. J Adv Pharm Technol Res. 2011;2:88–93.
- Otten A. The GATT TRIPS Agreement and health care in India. Natl Med J India. 1995;8:1–3.
- Thawani V, Gharpure K, Thawani M. Patent laws must be in the national interest. Indian J Pharmacol. 2006;38:70–2.
- http://www.Patentmatics.org/pub2005/pub10b.pdf on 13.10.2005 .
- http://www.Scitech.today.com/story.html?Story_id=31764on 27.03.2005 .
- http://www.cdsco.nic.in/html/scheduleY%20(Amended%20Version2005)%20original.htm .
- http://www.icmr.nic.in/ethical_guidelines.pdf .
- http://cdsco.nic.in/html/GCP1.html .
- Nundy S, Gulhati CM. A new colonialism – Conducting clinical trials in India. N Engl J Med. 2005;352:1633–6.
- Thatte UM, Bavdeka SB. Clinical Research in India: Great expectations. J Postgrad Med. 2008;54:318–23.]
- Ghooi RB. Trials and tribulations of clinical research teaching and training.PerspectClin Res. 2010;1:139–42.
- http://www.pharmabiz.com/NewsDetails.aspx?aid=83047&sid=1.
- http://acplgroupindia.co.in/pdf/45.pdf
- http://www.cdsco.nic.in/forms/list.aspx?lid=1833&Id=31
- http://www.cdsco.nic.in/forms/list.aspx?lid=1833&Id=31
- http://www.cdsco.nic.in/forms/list.aspx?lid=1833&Id=31
- http://www.cdsco.nic.in/forms/SearchMore.aspx?Id=32
- http://www.cdsco.nic.in/forms/list.aspx?lid=1887&Id=32
- http://www.drugscontrol.org/Bill%20No.%20LVIII%20of%202013.pdf
- http://www.prsindia.org/uploads/media/drugs%20and%20Cosmetics /SCR-Drugs%20and%20cosmetics.pdf
- http://www.pharmabiz.com/NewsDetails.aspx?aid=79557&sid=1
- http://www.cdsco.nic.in/forms/list.aspx?lid=1843&Id=31